| << Chapter < Page | Chapter >> Page > |
Plants are unique organisms that can absorb nutrients and water through their root system, as well as carbon dioxide from the atmosphere. Soil quality and climate are the major determinants of plant distribution and growth. The combination of soil nutrients, water, and carbon dioxide, along with sunlight, allows plants to grow.
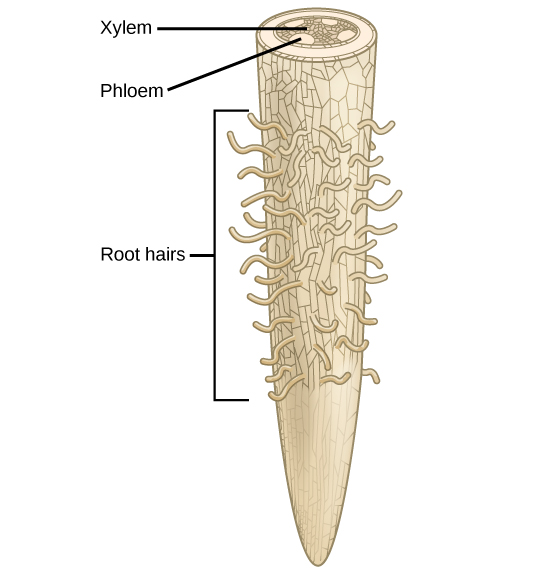
Since plants require nutrients in the form of elements such as carbon and potassium, it is important to understand the chemical composition of plants. The majority of volume in a plant cell is water; it typically comprises 80 to 90 percent of the plant’s total weight. Soil is the water source for land plants, and can be an abundant source of water, even if it appears dry. Plant roots absorb water from the soil through root hairs and transport it up to the leaves through the xylem. As water vapor is lost from the leaves, the process of transpiration and the polarity of water molecules (which enables them to form hydrogen bonds) draws more water from the roots up through the plant to the leaves ( [link] ). Plants need water to support cell structure, for metabolic functions, to carry nutrients, and for photosynthesis.
Plant cells need essential substances, collectively called nutrients, to sustain life. Plant nutrients may be composed of either organic or inorganic compounds. An organic compound is a chemical compound that contains carbon, such as carbon dioxide obtained from the atmosphere. Carbon that was obtained from atmospheric CO2 composes the majority of the dry mass within most plants. An inorganic compound does not contain carbon and is not part of, or produced by, a living organism. Inorganic substances, which form the majority of the soil solution, are commonly called minerals: those required by plants include nitrogen (N) and potassium (K) for structure and regulation.
Plants require only light, water and about 20 elements to support all their biochemical needs: these 20 elements are called essential nutrients ( [link] ). For an element to be regarded as essential , three criteria are required: 1) a plant cannot complete its life cycle without the element; 2) no other element can perform the function of the element; and 3) the element is directly involved in plant nutrition.
| Essential Elements for Plant Growth | |
|---|---|
| Macronutrients | Micronutrients |
| Carbon (C) | Iron (Fe) |
| Hydrogen (H) | Manganese (Mn) |
| Oxygen (O) | Boron (B) |
| Nitrogen (N) | Molybdenum (Mo) |
| Phosphorus (P) | Copper (Cu) |
| Potassium (K) | Zinc (Zn) |
| Calcium (Ca) | Chlorine (Cl) |
| Magnesium (Mg) | Nickel (Ni) |
| Sulfur (S) | Cobalt (Co) |
| Sodium (S) | |
| Silicon (Si) |
The essential elements can be divided into two groups: macronutrients and micronutrients. Nutrients that plants require in larger amounts are called macronutrients . About half of the essential elements are considered macronutrients: carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium and sulfur. The first of these macronutrients, carbon (C), is required to form carbohydrates, proteins, nucleic acids, and many other compounds; it is therefore present in all macromolecules. On average, the dry weight (excluding water) of a cell is 50 percent carbon. As shown in [link] , carbon is a key part of plant biomolecules.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?