| << Chapter < Page | Chapter >> Page > |
A convenient approach to determining E a for a reaction involves the measurement of k at different temperatures and using of an alternate version of the Arrhenius equation that takes the form of linear equation:
Thus, a plot of ln k versus gives a straight line with the slope from which E a may be determined. The intercept gives the value of ln A .
| T (K) | k (L/mol/s) |
|---|---|
| 555 | 3.52 10 −7 |
| 575 | 1.22 10 −6 |
| 645 | 8.59 10 −5 |
| 700 | 1.16 10 −3 |
| 781 | 3.95 10 −2 |
| ln k | |
|---|---|
| 1.80 10 −3 | −14.860 |
| 1.74 10 −3 | −13.617 |
| 1.55 10 −3 | −9.362 |
| 1.43 10 −3 | −6.759 |
| 1.28 10 −3 | −3.231 |
[link] is a graph of ln k versus To determine the slope of the line, we need two values of ln k , which are determined from the line at two values of (one near each end of the line is preferable). For example, the value of ln k determined from the line when is −2.593; the value when is −14.447.
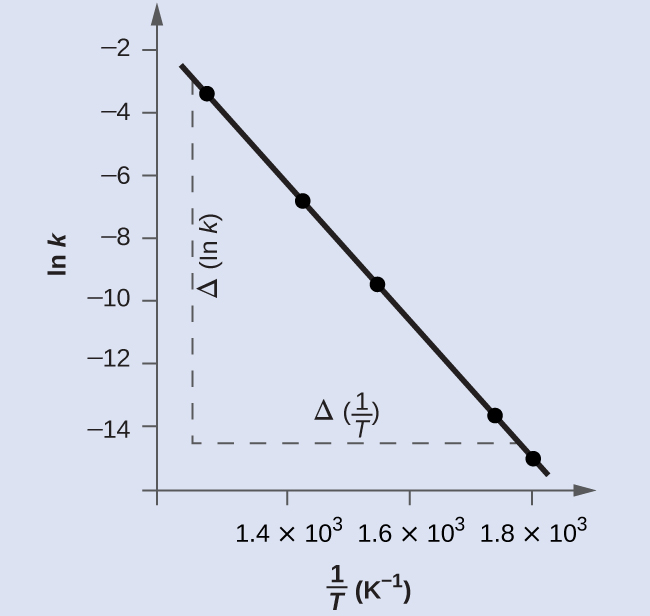
The slope of this line is given by the following expression:
Thus:
In many situations, it is possible to obtain a reasonable estimate of the activation energy without going through the entire process of constructing the Arrhenius plot. The Arrhenius equation:
can be rearranged as shown to give:
or
This equation can be rearranged to give a one-step calculation to obtain an estimate for the activation energy:
Using the experimental data presented here, we can simply select two data entries. For this example, we select the first entry and the last entry:
| T (K) | k (L/mol/s) | ln k | |
|---|---|---|---|
| 555 | 3.52 10 −7 | 1.80 10 −3 | −14.860 |
| 781 | 3.95 10 −2 | 1.28 10 −3 | −3.231 |
After calculating and ln k , we can substitute into the equation:
and the result is E a = 185,900 J/mol.
This method is very effective, especially when a limited number of temperature-dependent rate constants are available for the reaction of interest.
Assuming the kinetics of this reaction are consistent with the Arrhenius equation, calculate the activation energy for this decomposition.
113,000 J/mol
Chemical reactions require collisions between reactant species. These reactant collisions must be of proper orientation and sufficient energy in order to result in product formation. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The Arrhenius equation describes the relation between a reaction’s rate constant and its activation energy, temperature, and dependence on collision orientation.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?