| << Chapter < Page | Chapter >> Page > |
The boron atom in B(OH) 3 is sp 2 hybridized and is located at the center of an equilateral triangle with oxygen atoms at the corners. In solid B(OH) 3 , hydrogen bonding holds these triangular units together. Boric acid, shown in [link] , is a very weak acid that does not act as a proton donor but rather as a Lewis acid, accepting an unshared pair of electrons from the Lewis base OH − :
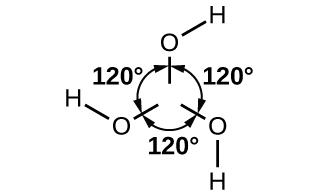
Heating boric acid to 100 °C causes molecules of water to split out between pairs of adjacent –OH groups to form metaboric acid, HBO 2 . At about 150 °C, additional B-O-B linkages form, connecting the BO 3 groups together with shared oxygen atoms to form tetraboric acid, H 2 B 4 O 7 . Complete water loss, at still higher temperatures, results in boric oxide.
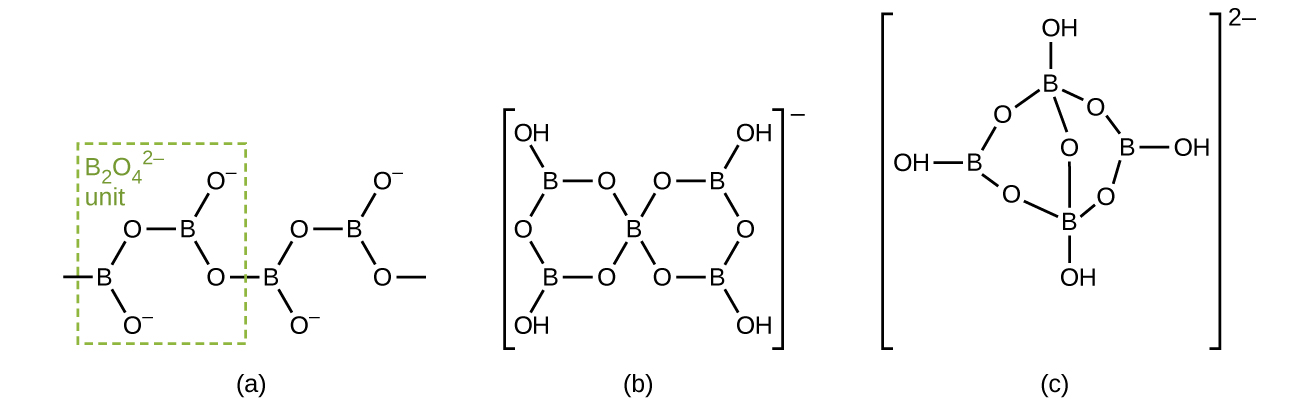
Borates are salts of the oxyacids of boron. Borates result from the reactions of a base with an oxyacid or from the fusion of boric acid or boric oxide with a metal oxide or hydroxide. Borate anions range from the simple trigonal planar ion to complex species containing chains and rings of three- and four-coordinated boron atoms. The structures of the anions found in CaB 2 O 4 , K[B 5 O 6 (OH) 4 ]⋅2H 2 O (commonly written KB 5 O 8 ⋅4H 2 O) and Na 2 [B 4 O 5 (OH) 4 ]⋅8H 2 O (commonly written Na 2 B 4 O 7 ⋅10H 2 O) are shown in [link] . Commercially, the most important borate is borax, Na 2 [B 4 O 5 (OH) 4 ]⋅8H 2 O, which is an important component of some laundry detergents. Most of the supply of borax comes directly from dry lakes, such as Searles Lake in California, or is prepared from kernite, Na 2 B 4 O 7 ⋅4H 2 O.
Silicon dioxide, silica, occurs in both crystalline and amorphous forms. The usual crystalline form of silicon dioxide is quartz, a hard, brittle, clear, colorless solid. It is useful in many ways—for architectural decorations, semiprecious jewels, and frequency control in radio transmitters. Silica takes many crystalline forms, or polymorphs , in nature. Trace amounts of Fe 3+ in quartz give amethyst its characteristic purple color. The term quartz is also used for articles such as tubing and lenses that are manufactured from amorphous silica. Opal is a naturally occurring form of amorphous silica.
The contrast in structure and physical properties between silicon dioxide and carbon dioxide is interesting, as illustrated in [link] . Solid carbon dioxide (dry ice) contains single CO 2 molecules with each of the two oxygen atoms attached to the carbon atom by double bonds. Very weak intermolecular forces hold the molecules together in the crystal. The volatility of dry ice reflect these weak forces between molecules. In contrast, silicon dioxide is a covalent network solid. In silicon dioxide, each silicon atom links to four oxygen atoms by single bonds directed toward the corners of a regular tetrahedron, and SiO 4 tetrahedra share oxygen atoms. This arrangement gives a three dimensional, continuous, silicon-oxygen network. A quartz crystal is a macromolecule of silicon dioxide. The difference between these two compounds is the ability of the group 14 elements to form strong π bonds. Second-period elements, such as carbon, form very strong π bonds, which is why carbon dioxide forms small molecules with strong double bonds. Elements below the second period, such as silicon, do not form π bonds as readily as second-period elements, and when they do form, the π bonds are weaker than those formed by second-period elements. For this reason, silicon dioxide does not contain π bonds but only σ bonds.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?