| << Chapter < Page | Chapter >> Page > |
What did they discover? Most particles passed right through the foil without being deflected at all. However, some were diverted slightly, and a very small number were deflected almost straight back toward the source ( [link] ). Rutherford described finding these results: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you” Ernest Rutherford, “The Development of the Theory of Atomic Structure,” ed. J. A. Ratcliffe, in Background to Modern Science , eds. Joseph Needham and Walter Pagel, (Cambridge, UK: Cambridge University Press, 1938), 61–74. Accessed September 22, 2014, https://ia600508.us.archive.org/3/items/backgroundtomode032734mbp/backgroundtomode032734mbp.pdf. (p. 68).
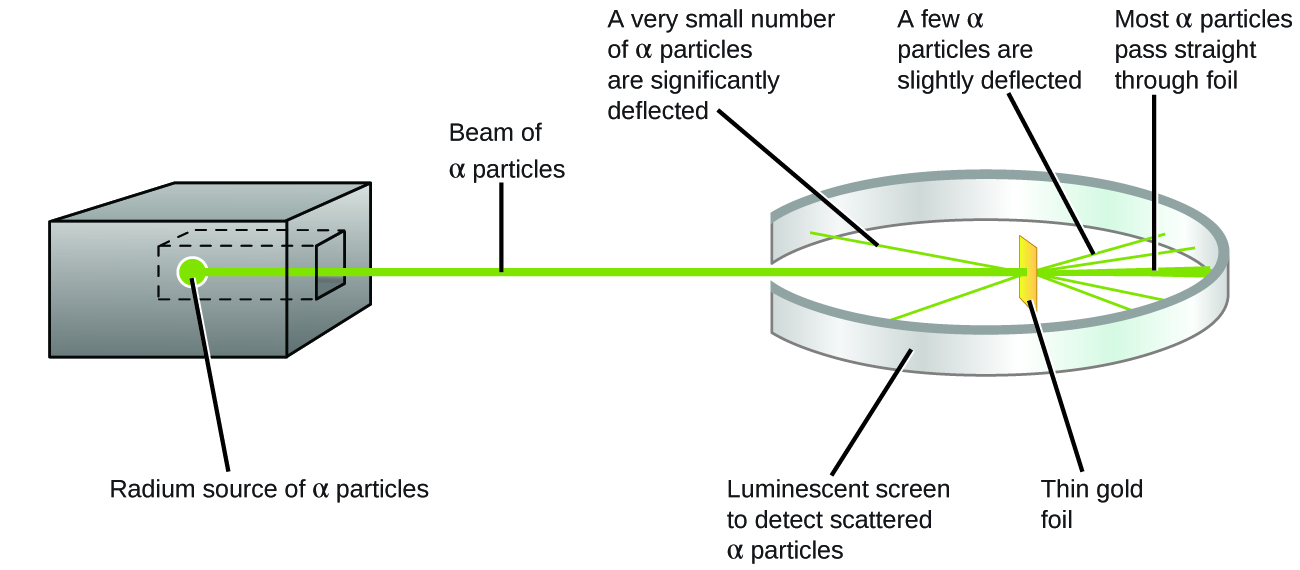
Here is what Rutherford deduced: Because most of the fast-moving α particles passed through the gold atoms undeflected, they must have traveled through essentially empty space inside the atom. Alpha particles are positively charged, so deflections arose when they encountered another positive charge (like charges repel each other). Since like charges repel one another, the few positively charged α particles that changed paths abruptly must have hit, or closely approached, another body that also had a highly concentrated, positive charge. Since the deflections occurred a small fraction of the time, this charge only occupied a small amount of the space in the gold foil. Analyzing a series of such experiments in detail, Rutherford drew two conclusions:
View this simulation of the Rutherford gold foil experiment. Adjust the slit width to produce a narrower or broader beam of α particles to see how that affects the scattering pattern.
This analysis led Rutherford to propose a model in which an atom consists of a very small, positively charged nucleus, in which most of the mass of the atom is concentrated, surrounded by the negatively charged electrons, so that the atom is electrically neutral ( [link] ). After many more experiments, Rutherford also discovered that the nuclei of other elements contain the hydrogen nucleus as a “building block,” and he named this more fundamental particle the proton , the positively charged, subatomic particle found in the nucleus. With one addition, which you will learn next, this nuclear model of the atom, proposed over a century ago, is still used today.


Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?