| << Chapter < Page | Chapter >> Page > |
Plan an experiment using a potato cannon, meter stick, and pressure gauge to measure the work done by a potato cannon. Your experiment should produce P–V diagrams to analyze and determine the work done on a gas or by a gas. What do you need to measure? How will you measure it? Can you modify the potato cannon to make your measurements easier? When you perform multiple trials, what variables do you need to keep fixed between each trial? Which variables will you change?
One class decides to use a heavy piston, capable of being latched in place, to replace the potato. They latch the piston in place so that the contained volume is 0.50 L, load the cannon with fuel, and close the cannon with their pressure gauge, which reads 101 kPa. Then they light the fuel, and the pressure jumps to 405 kPa. Next, they release the latch, and the piston moves out until the internal volume is 2.0 L. The pressure is measured at this point to be 101 kPa again. Finally, they release the pressure gauge, and move the piston back down to 0.50 L, still at atmospheric pressure. Draw a diagram of this process, and calculate the net work performed by this system. Can you think of any ways to improve the measurements?
You should find that you have a right triangle on a P–V diagram, the area of which is the net work done by the system. Using a pressure gauge that can take continuous measurements during the expansion phase might be useful, as it is unlikely that this would actually be a linear process.
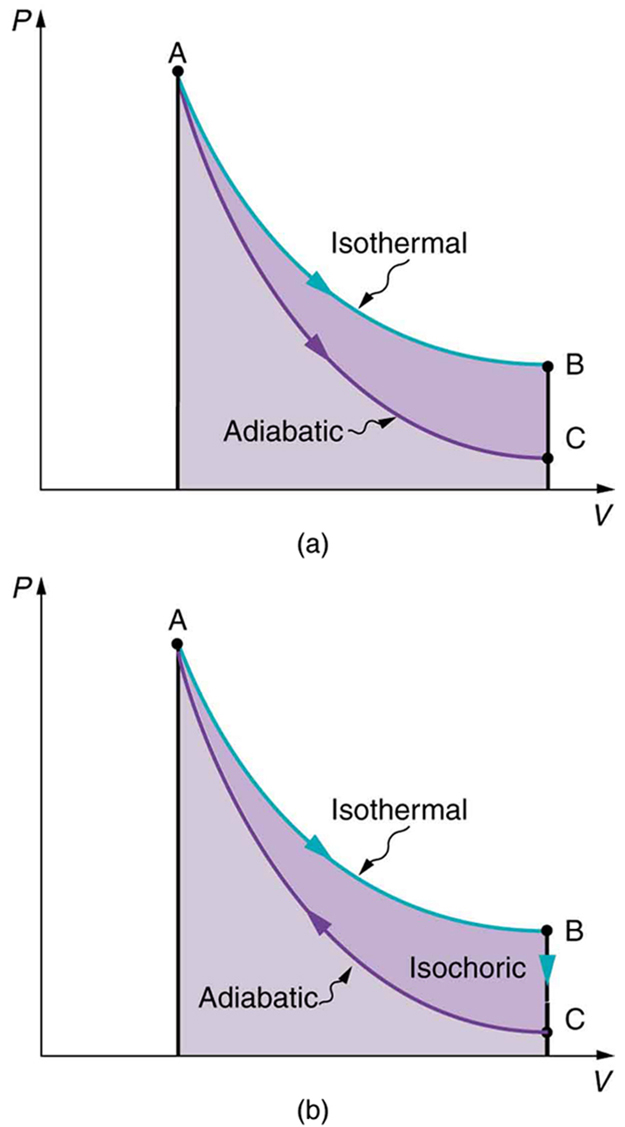
Both isothermal and adiabatic processes such as shown in [link] are reversible in principle. A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the reverse path. The reverse isothermal and adiabatic paths are BA and CA, respectively. Real macroscopic processes are never exactly reversible. In the previous examples, our system is a gas (like that in [link] ), and its environment is the piston, cylinder, and the rest of the universe. If there are any energy-dissipating mechanisms, such as friction or turbulence, then heat transfer to the environment occurs for either direction of the piston. So, for example, if the path BA is followed and there is friction, then the gas will be returned to its original state but the environment will not—it will have been heated in both directions. Reversibility requires the direction of heat transfer to reverse for the reverse path. Since dissipative mechanisms cannot be completely eliminated, real processes cannot be reversible.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?